Writen by Sasinthiran
Edited by Xin Chen, Yingchen and Keshiniy
“How on earth are you ever going to explain in terms of chemistry and physics so important a biological phenomenon as first love?”
–Albert Einstein

While science has taught us much about the complexities of human behaviour, nothing seems to be as enigmatic as the concept of love. Love is such a powerful force that it has driven some to start great wars while inspiring others to produce marvelous works of art, poems, songs and novels. It is both rewarding and punishing, is often unpredictable and drives one to act in irrational, sometimes ridiculous ways. Einstein might be right in that this complex concept of love cannot be simply reduced to basic principles in science. However, in light of Valentine’s Day, let us review some of the insights neuroscience research has given us on how the brain handles matters of the heart.
Love, sex and other drugs
When falling in love, most people experience a rise in the levels of the stress hormone cortisol (a corticosteroid) which helps in overcoming the initial neophobia characteristic of the initial phases of starting a relationship with someone special (de Boer, et al., 2012). Many would also relate to experiencing changes in sleep patterns, a general loss of appetite and occasional mood swings when falling in love. These changes are an effect of depleting Serotonin (a neurotransmitter in the brain) levels which are inversely correlated with the levels of corticosteroids.
The brain has evolved several mechanisms to keep the passion burning after the initial period of falling in love. Most people will find it difficult to accept the idea that love is in fact an addiction. The brain is addicted to pleasure and positive affect, more specifically, it craves any activity that leads to the activation of dopaminergic pathways which trigger the brain’s reward response with the release of neurotransmitter dopamine (Insel, 2003). This pathway, known as the mesocorticolimbic pathway (see image below), originates in the ventral tegmental area (VTA) which project to the nucleus accumbens which in turn projects to the ventral pallidum and thalamus (midbrain structures as labelled below). Neurons from the thalamus then project to the prefrontal and cingulate cortex (PFC) (Everitt & Wolfe, 2002).

Research shows that addictive drugs such as cocaine are in fact addictive primarily because the activate such pathways leading to a reward response, leaving the brain craving for more (Koob & Moal, 1997). In other words, the more a particular behaviour such as taking cocaine triggers the reward centres of the brain, the more the brain seeks out such behaviour through learned operant conditioning by positive reinforcement. This then increases the occurrence of that behaviour in the future (addiction). Interestingly, in an fMRI study whereby participants were shown pictures of people they ‘liked’ versus people they ‘loved’, it was shown that the latter group elicited a greater activation of the brain’s reward pathways (Bartels & Zeki, 2000). Hence, being in love , just like drugs, triggers the same reward pathways of the brain and this leaves the brain craving for more due to positive reinforcement.
The effects of Oxytocin (OT) and arginine Vasopressin (AVP) have been extensively studied in bonding studies involving voles, comparing those that by their own nature, have monogamous relationships (prairie voles) with those that are polygamous (montane voles) (Young & Wang, 2004; Lim & Young, 2006). From such studies, it was found that blocking the release of these two hormones caused the prairie voles to be promiscuous while injection of the hormones into these voles caused them to be faithful to their partners, even when they were prevented from having sex. It would then be reasonable to expect that injecting the hormones into promiscous individuals could reduce this behaviour (many have actually suggested this as a ‘cure’ for human promiscuity!). However, injection of the hormones into montane voles which are promiscuous by nature, did not render them monogamous and it was found that the reason for this was that montane voles did not have a sufficient number of receptors for these hormones in the reward centres of their brains (Edwards & Self, 2006). Hence, they were not responsive to the reinforcing effects of oxytocin and vasopressin. This possibly suggests that certain individuals lacking the receptors for these hormones (and hence not responsive to them) are actually predisposed to exhibit promiscuity!
Oxytocin and Vasopressin are both synthesized in the brain’s hypothalamic paraventricular and supraoptic nuclei and secreted by the posterior pituitary into circulation. Both neuropeptide hormones are known to released during breastfeeding, child birth and sexual stimulation and have a neuromodulatory effect on different regions of the brain (Insel, 2010). Some of these brain regions are involved in regulating social behaviour which is then modulated as a result. Oxytocin in particular, reduces fear and anxiety related to social situations by reducing amygdala activity (Neumann, et al., 2000; Kirsch, et al., 2005), enhances social memory (Insel, 2010) and activates reward circuits in the brain involving dopamine release (Lim & Young, 2006). In fact, Oxytocin receptors have been identified in the nucleus accumbens (Insel & Shapiro, 1992) and the V1a Vasopressin receptor has been identified in the ventral pallidum (Insel, et al., 1994), both of which are part of the mecocorticolimbic pathway described earlier. Ultimately these hormones help modulate social behaviours favouring the long term maintenance of monogamous relationships through operant conditioning by rewarding such pro-social behaviours via brain’s reward system (Insel, 2010).
Quite predictably, couples with higher blood plasma oxytocin levels were found to have more positive communication behaviours (Gouin, et al., 2010), greater perceived spousal support and a higher frequency of hugs and massages (Grewen, et al., 2005). Research has also found that an individual becomes a stronger learned stimuli for oxytocin release in their partners with each successive sexual encounter with that person (Witt, 1997), proving that sex itself can be addictive. This then helps to facilitate a deeper bond between the sexual partners and increase the likelihood that they will stay together, which from an evolutionary perspective, will be essential for raising a child since the act of copulation eventually leads to begetting an offspring.
Interestingly, it seems that love can even be affected at the genetic level. High levels of a polymorphic variant of the V1a Vasopressin receptor gene, with a variation in the RS3 344 section, has been found to correlate with lower partner bonding, higher incidences of marital crises within a year and an increased likelihood of cohabitating as compared to being married to a partner in a self-report study that studied men in long-term relationships (Walum, et al., 2008).
The Social Brain
The Belongingness Hypothesis states that people have a pervasive need to form and maintain significant interpersonal relationships with others (Baumeister & Leary, 1995). In fact, research shows that the need for social interaction may be more profoundly felt than other basic needs such as hunger (Baumeister & Leary, 1995; Cacioppo, et al., 2000). Socialising with others was an essential skill for survival and reproduction in the hunter-gatherer days of human history (Baumeister & Leary, 1995). The brain has evolved over the years to become a highly social organ with specific neural networks that have been perfected over thousands of years of evolution to support this function.
One of the ways the brain has evolved to support social bond formation is by differentiating the cognitive processing of information pertaining to significant others as compared to general acquaintances. Memories and information relating to those with whom we share a significant personal relationship is processed in a unique person-by-person basis while cognitive processes for other acquaintances are stored, organised and processed on the basis of general attributional categories such as preferences, traits and duties instead of person categories (Pryor & Ostrom, 1981; Ostrom, et al., 1993). One might hypothesize that the purpose for such a distinction in cognitive processing might be to improve the speed and efficacy of the recall of information pertaining to significant people in our lives. In fact, it was reported that recall for information about both groups of people did not actually differ significantly. However, another possibility is that the specific neural networks catered to processing information about significant people may also project to emotion processing areas of the brain and thus allow for the addition of an extra layer (emotion) to the retrieval of stored information about them since relationships and attachment are closely tied to affect. This could also explain why recalling information about significant people often triggers positive emotions.
Love is blind and unconditional
The frontal cortex is the central executive centre of the brain that processes higher cognitive functions such as logical thinking, judgements, decision making and morality. In an fMRI study it was found that brain areas associated with negative emotional processing (the parietal cortex and temporal lobe) as well as other areas involved in assessing the emotions and intentions of other and other aspects of social judgements (the frontal cortex) were less active when viewing pictures of people they ‘loved’ versus pictures of their friends (Bartels & Zeki, 2000).
It then comes as no surprise that people in a relationship tend to demonstrate a self-serving bias when interpreting their partner’s outcomes in an experiment by giving them credit if they succeeded and not attributing blame to them when they fail (Fincham, et al., 1987). They also demonstrate this bias in giving their partners a more favourable interpretation of their role in causing events (causal attribution) (Craig, 1991). This probably relates to why we tend to overlook the flaws of those we are smitten over as this is probably an adaptation of the brain to aid in maintaining an existing relationship.
Researchers have also found that activity in the amygdala which is associated with fearful situations, is reduced when viewing pictures of their partners (Zeki, 2007). The suppression of judgment and increase in trust as a result of diminished fear (amygdala activity suppression) leads to increased bonding between partners and may also account for the irrational behaviour of people in love.
Rejections are painful – literally
Some of us might have had the unfortunate experience of a break-up and we know that it can be an unpleasant experience. What is interesting though, is that the brain perceives the pain of social rejection the same way it would physical pain. This has been demonstrated in fMRI studies which have shown that the same brain areas, such as the anterior cingulate cortex (ACC), anterior insula (AI) and the right ventral prefrontal cortex (RVPFC), that are activated when processing the ‘affective’ or unpleasant component of physical pain, are also activated in response to social rejection (Eisenberger, et al., 2003). In fact, in one fMRI study it was found that presenting participants a picture of their ex-partners who rejected them not only activated the ACC but also triggered activity in the regions of the somatosensory cortex such as S2 which respond directly to physical sensations of pain (Kross, et al., 2011).
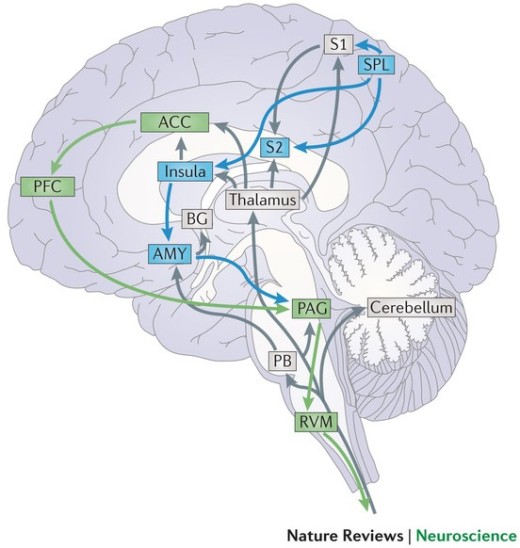
It has been suggested that social system of the brain may have evolved to rely on the neural pathways for processing physical pain to indicate when social relationships are threatened, given that social connections are important for human survival (Panksepp, 1998). This further lends support to the role of operant conditioning in helping to maintain relationships. An individual learns the appropriate behaviours that will keep a relationship healthy through positive reinforcement in terms of triggering reward pathways of the brain that encourages future occurrence of such behaviour, as well as through positive punishment in terms of the pain associated with social rejection in response to behaviours that threaten relationships (Eisenberger, 2011).
The rules of attraction
An experiment involving the use of PET (positron emission tomography) to measure regional cerebral blood flow (rCBF) as a means of identifying brain areas that are more active (with more cerebral blood flow), identified that increased activity in the left insula correlated with reporting of the attractiveness of unfamiliar faces (Nakamura, et al., 1999). In what seems to be eerily similar to mind control, researchers have demonstrated that judgements of physical attractiveness can be manipulated by evoking different emotions in participants through music (May & Hamilton, 1980). It was found that evoking positive affect such that the brain’s reward pathways are activated through rock music increased perceptual judgements of attractiveness.
Anthropological research has shown that since the stone ages, people will select mates who stand out from the rest of the crowd when presented with a choice of mates of equal value (Frost, 2006). Researchers have found that the mere exposure effect, a principle in which regular exposure to a neutral of positive stimuli generally increases the liking for that stimuli (Zajonc, 1968) can be applicable to humans as well (Swap, 1977). This probably explains why some men finally win over the woman they court after some time. Either that or the women are just playing hard to get! Studies have shown that sharing a meal has a profound effect on human bonding as higher levels of oxytocin release has been measured in such settings (Wittig, et al., 2014). Hence, a nice romantic dinner on the first date might not be a bad idea after all.
In romantic situations, males use more uncommon, fancy words than they do in other situations (Rosenberg & Tunney, 2008). This could serve as a litmus test for knowledgeable women to identify if someone is trying to impress them! In one study, higher levels of fertility in women was found to be associated with lower levels of linguistic matching in their male partners in experimental setting (Coyle & Kaschak, 2012). Linguistic alignment is usually used to signal affiliation (Giles, et al., 1991) and leads to increased liking between participants in social interactions (Chartrand & Bargh, 1999; Cheng & Chartrand, 2003). However, it was noted that men paired with women in the fertile stages of their menstrual cycle, chose to use different syntactic structures in their speech and not mimic that of their partners (non-conforming behaviour) as one would expect. The men seemed to have picked up subtle subconscious cues about the female partner’s fertility and coud be presenting their non-conforming speech behaviour as a display of their fitness as a mate.
Having a sense of humour is a common feature that both men and women look for in their ideal mates, albeit with a significant difference. Men prefer women who appreciate their jokes, and not necessarily women who are funny themselves, while women prefer men who made them laugh (Bressler & Balshine, 2006). It was also observed from the study that women signal their level of attraction to their partners by the frequency of their laughter while men’s laughter was not correlated to their degree of attraction to a potential mate.
Studies have shown that men and women in a romantic relationship share fundamental differences in areas of the brain that are more active. Men, have been found to have increased activity in areas of the brain involved in integrating visual stimuli (Narumoto, et al., 2001) while women have greater activation in areas associated with memory, attention and emotion (Gray, et al., 2002; Maddock, et al., 2003; Velanova, et al., 2003). This is supported by the evolutionary view of gender priorities in looking for a mate as males look for healthy mothers to carry their child while females look for possible security and resources offered by a male mate in raising her child (Fisher, 2004).
Through better or worse
In one particular fMRI study, women who held their husband’s hands were found to have reduced activity in parts of their brain involved with processing the emotional and arousing aspects of pain when they were told to anticipate an electric shock, as compared to women who were alone (Coan, et al., 2006). Interestingly, it was found that the reduction in pain-associated activity correlated with the quality of their marriages such that happily married women had a greater degree of reduction in activity. Another observation from the study was that women who held the hands of men they did not know also showed a reduction of activity in pain processing, albeit a smaller reduction than the women who held their husband’s hands. This shows that love, or any relationship for that matter, may have a protective effect on the brain in terms of reducing the processing of unpleasant or noxious stimuli.
Conclusion
As reviewed in this article, neuroscience research may provide some insight, and at best, a fragmented view on neurological basis for some behaviours demonstrated by people in love. However, love is a complex emergent behaviour which we can never fully appreciate and artificially re-create even with the advances in knowledge.
References
Bartels, A. & Zeki, S., 2000. The Neural Basis of Romantic Love. Neuroreport, 11(17), pp. 3829-3834.
Baumeister, R. F. & Leary, M. R., 1995. The Need to Belong: Desire for Interpersonal Attachments as a Fundamental Human Motivation. Psychological Bulletin, 117(3), pp. 497-529.
Bressler, E. R. & Balshine, S., 2006. The Influence of Humor on Desirability. Evolution and Human Behavior, 27(1), pp. 29-39.
Bushnell, M.C., Ceko, M., Low, L. A., 2013. Cognitive and Emotional Control of Pain and its Distribution in Chronic Pain. Nature Reviews Neuroscience, Volume 14, pp. 502-511.
Cacioppo, J. T., Berntson, G. G., Sheridan, J. F. & McClintock, M. K., 2000. Multilevel Integrative Analyses of Human Behavior Social Neuroscience and the Complementing Nature of Social and Biological Approaches. Psychological Bulletin, 126(6), pp. 829-843.
Chartrand, T. L. & Bargh, J. A., 1999. The Chameleon Effect: The Perception-behavior link and social interaction. Journal of Personality and Social Psychology, Volume 76, pp. 893-910.
Cheng, C. M. & Chartrand, T. L., 2003. Self-monitoring Without Awareness: Using Mimicry as a Nonconscious Affiliation Strategy. Journal of Personality and Social Psychology, Volume 85, pp. 1170-1179.
Coan, J. A., Schaefer, H. S. & Davidson, R. J., 2006. Lending a Hand: Social Regulation of the Neural Response to Threat. Psychological Sciecne, 17(2), pp. 1032-1039.
Coyle, J. M. & Kaschak, M. P., 2012. Female Fertility Affects Men’s Linguistic Choices. PLoS One, 7(2).
Craig, A. A., 1991. How People Think About Causes: Examination of the Typical Phenomenal Organization of Attributions for Success and Failure. Social Cognition, 9(4), pp. 295-329.
de Boer, A., van Buel, E. M. & Ter Horst, G. J., 2012. Love Is More Than Just A Kiss: A Neurobiological Perspective On Love and Affection. Neuroscience, Volume 201, pp. 114-124.
Edwards, S. & Self, D. W., 2006. Monogamy: Dopamine Ties the Knot. Nature Neuroscience, Volume 9, pp. 7-8.
Eisenberger, N. I., 2011. Why Rejection Hurts: What Social Neuroscience Has Revealed About the Brain’s Response to Social Rejection. In: M. Brockman, ed. Future Science 19 Essays from the Cutting Edge. New York: Vintage Books, pp. 170-173.
Eisenberger, N. I., Lieberman, M. D. & Williams, K. D., 2003. Does Rejection Hurt? An fMRI Study of Social Exclusion. Science, Volume 302, pp. 290-292.
Everitt, B. J. & Wolfe, M. E., 2002. Psychomotor Stimulant Addiction: A Neural Systems Perspecive. Journal of Neuroscience, Volume 22, pp. 3312-3320.
Fincham, F. D., Beach, S. R. & Baucom, D. H., 1987. Attribution processes in distressed and nondistressed couples: IV. Self–partner attribution differences.. Journal of Personality and Social Psychology, 52(4), pp. 739-748.
Fisher, H., 2004. Why We Love: The Nature and Chemistry of Romantic Love. New York: s.n.
Frost, P., 2006. European Hair and Eye Color A case of Frequency-Dependent Sexual Selection. Evolution and Human Behavior, 27(2), pp. 85-103.
Giles, H., Coupland, N. & Coupland, J., 1991. Accomodation Theory: Communication, Context, and Consequence. In: H. Giles, N. Coupland & J. Coupland, eds. Contexts of Accommodation: Developments in Applied Psycholinguistics. Cambridge: Cambridge University Press, pp. 1-68.
Gouin, J. et al., 2010. Marital Behavior, Oxytocin, Vasopressin, and Wound Healing. Psychoneuroendocrinology, 35(7), pp. 1082-1090.
Gray, J. R., Braver, T. S. & Raichle, M. E., 2002. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 99(6), pp. 4115-4120.
Grewen, K. M., Girdler, S. S., Amico, J. & Light, K. C., 2005. Effects of Partner Support on Resting Oxytocin, Cortisol, Norepinephrine, and Blood Pressure Before and After Warm Partner Contact. Psychosomatic Medicine, 67(4), pp. 531-538.
Insel, T. R., 2003. Is Social Attachment an Addictive Disorder?. Physiology and Behavior, 79(3), pp. 351-357.
Insel, T. R., 2010. The Challenge of Translation in Social Neuroscience: A Review of Oxytocin, Vasopressin, and Affiliative Behavior. Neuron, Volume 65, pp. 768-779.
Insel, T. R. & Shapiro, L. E., 1992. Oxytocin Receptor Distribution Reflects Social Organization in Monogamous and Ploygamous Voles. Proceedings of the National Academy of Sciences of the United States of Americaa, Volume 89, pp. 5981-5985.
Insel, T. R., Wang, Z. & Ferris, C. F., 1994. Patterns of Vasopressin Receptor Distribution Associated with Social Organization in Monogamous and Non-monogamous microtine rodents. Journal of Neuroscience, Volume 14, pp. 5381-5392.
Kirsch, P. et al., 2005. Oxytocin Modulates Neural Circuitry for Social Cognition and Fear in Humans. Journal of Neuroscience, Volume 25, pp. 11489-11493.
Koob, G. F. & Moal, M. L., 1997. Drug Abuse: Hedonic Homeostatic Dysregulation. Science, Volume 278, pp. 52-58.
Kross, E. et al., 2011. Social Rejection Shares Somatosensory Representations with Physical Pain. Proceedings of the National Academy of Sceinces of the United States of America, 108(15), pp. 6270-6275.
Lim, M. M. & Young, L. J., 2006. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior, 50(4), pp. 506-517.
Lim, M. M. & Young, L. J., 2006. Neuropeptidergic Regulation of Affiliative Behavior and Social Bonding in Animals. Hormones and Behavior, Volume 50, pp. 506-517.
Maddock, R. J., Garrett, A. S. & Buonocore, M. H., 2003. Posterior Cingulate Cortex Activation by Emotional Words: fMRI Evidence From a Valence Decision Task. Human Brain Mapping, 18(1), pp. 30-41.
May, J. L. & Hamilton, P. A., 1980. Effects of musically evoked affect on women’s interpersonal attraction toward and perceptual judgments of physical attractiveness of men. Motivation and Emotion, 4(3), pp. 217-228.
Nakamura, K. K. R. et al., 1999. Activation of the Right Inferior Frontal Cortex During Assessment of Facial Emotion. Journal of Neurophysiology, 82(3), pp. 1610-1614.
Narumoto, J. et al., 2001. Attention to Emotion Modulates fMRI Activity in Human Right Superior Temporal Sulcus. Brain Research. Cognitive Brain Research, 12(2), pp. 225-231.
Neumann, I. D., Torner, L. & Wigger, A., 2000. Brain Oxytocin: Differential Inhibition of Neuroendocrine Stress Responses and Anxiety-related Behaviour in Virgin, Pregnant and Lactating Rats. Neuroscience, Volume 95, pp. 567-575.
Ostrom, T. M., Carpenter, S. L., Sedikides, C. & Li, F., 1993. Differential Processing of In-Group and Out-Group Information. Journal of Personality ad Social Psychology, 64(1), pp. 21-34.
Panksepp, J., 1998. Affective Neuroscience. New York: Oxford University Press.
Pryor, J. B. & Ostrom, T. M., 1981. The cognitive organization of social information: A converging-operations approach. Journal of Personality and Social Psychology, 41(4), pp. 628-641.
Puce, A., Allison, T., Gore, J. C. & McCarthy, G., 1995. Face-Sensitive Regions in Human Extrastriate Cortex Studied by Functional MRI. Journal of Neurophysiology, 74(3), pp. 1192-1199.
Rosenberg, J. & Tunney, R. J., 2008. Human Vocabulary Use as Display. Evolutionary Psychology, Volume 6, pp. 538-549.
Swap, W. C., 1977. Interpersonal Attraction and Repeated Exposure to Rewarders and Punishers. Social Psychology Bulletin, 3(2), pp. 248-251.
Velanova, K. et al., 2003. Functional-anatomic Correlates of Sustained and Transient Processing Components Engaged During Controlled Retrieval. Journal of Neuroscience, 23(24), pp. 8460-8470.
Walum, H. et al., 2008. Genetic Variation in the Vasopressin Receptor 1a Gene (AVPR1A) Associates with Pair-bonding Behavior in Humans. Proceedings of the National Academy of Sciences of the United States of America, 105(37), pp. 14153-14156.
Witt, D. M., 1997. Regulatory Mechanisms of Oxytocin-Mediated Sociosexual Behavior. Annals of the New York Academy of Sciences, Volume 807, pp. 287-301.
Wittig, R. M. et al., 2014. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proceedings of the Royal Society B Biological Sciences, 281(1778), pp. 1-10.
Young, L. J. & Wang, Z., 2004. The Neurobiology of Pair Bonding. Nature Neuroscience, Volume 7, pp. 1048-1054.
Zajonc, R. B., 1968. Attitudinal Effects of Mere Exposure. Journal of Personality and Social Psychology, 9(2), pp. 1-27.
Zeki, S., 2007. The Neurobiology of Love. FEBS Letters, 581(14), pp. 2575-2579.
